
Transition metals – Periodic tables have an extra block of transition metals at the bottom, for elements called rare-earths (or lanthanides) and actinides. The groups of elements with similar properties have names and are normally coloured differently in a periodic table. The more you find out about atomic structure, the more patterns and relationships you’ll find in the periodic table. Other patterns are there, too, For example, the energy needed to get an electron away from an atom: Atomic number increases as you move down a column.Atomic number increases as you move right along a row.

You’ll notice that elements in the periodic table are arranged in rows and columns.Įlements in a group (column) have the same number of outer electrons, so they have similar chemical properties. For example, the square for iron will look a bit like this: In some versions of the table, these squares can contain a lot of writing, but to start with, three pieces of information are enough – each square should contain the name of the element, its official chemical symbol and its atomic number. It will help to have a copy of the periodic table – the one here will get you started, or you can print a full one from the website below.Įach element has a square in the periodic table. The element iron is made only of iron atoms, and iron atoms are the same everywhere – iron atoms on Earth are the same as iron atoms on Mars. Elements cannot be split into simpler substances using normal chemical methods. ElementsĪn element is a substance that consists of atoms with the same atomic number. From this, we can work out how the electrons are arranged, and this will tell us how an element will react with others. The atomic number can also tell us how many electrons an atom has.
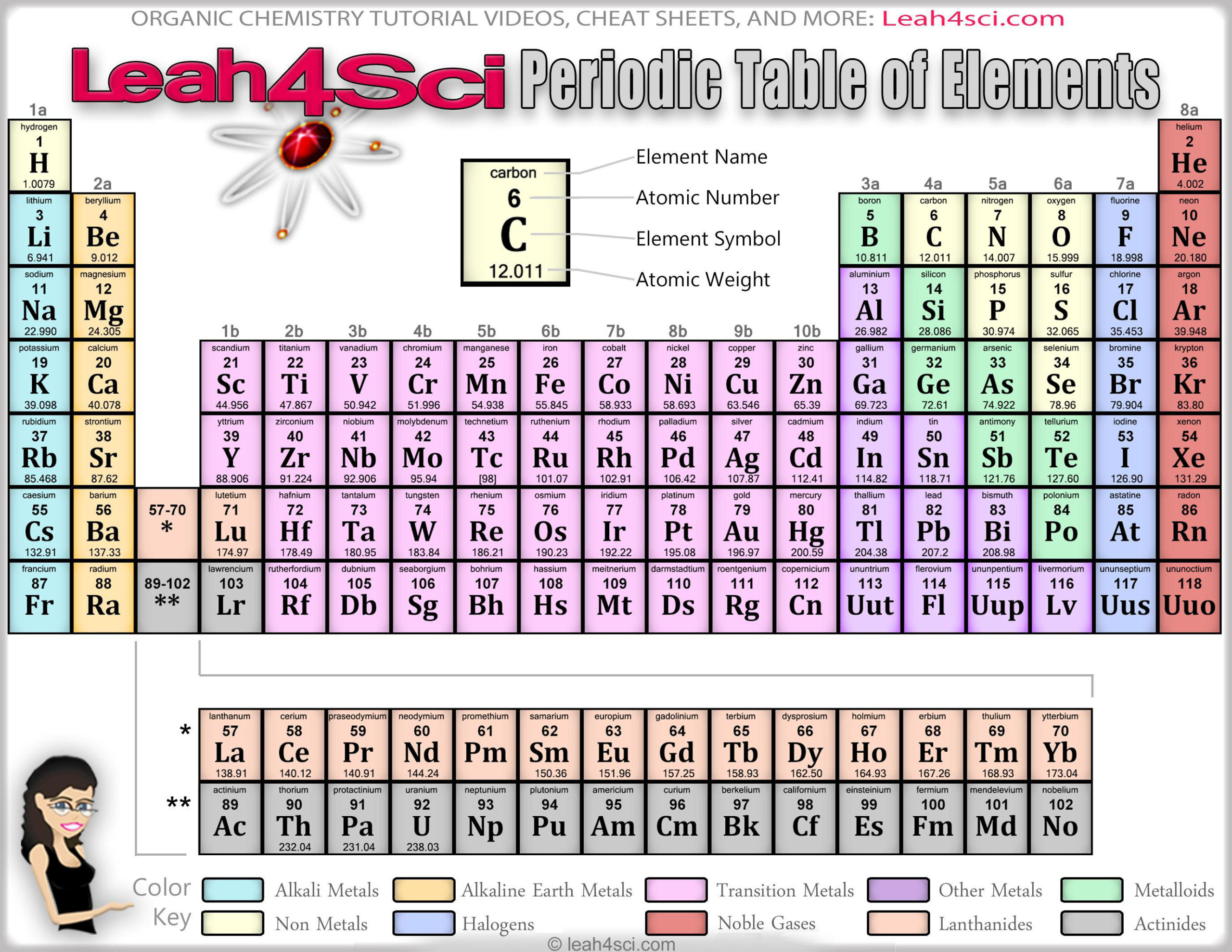
For example, if an atom has six protons, it can only be carbon. The atomic number of an element is the number of protons an atom has – the number of protons determines what an element is. The electrons are organised in energy levels – how an element behaves depends on how easy it is to gain or lose electrons in the outermost energy levels. Electrons move around within a large area of space outside the nucleus. Protons and neutrons form the nucleus at the centre of an atom (hydrogen is a bit different, it only has a proton). There are smaller particles, but they don’t concern us here. An atom has three basic parts – protons, neutrons and electrons.


 0 kommentar(er)
0 kommentar(er)
